Reusable medical devices need to have validated cleaning instructions to ensure patient safety and minimize health-care-acquired infections, corrective actions, recalls, and to ensure compliance with current regulatory standards. For devices that need to comply with US FDA, the requirements regarding the setup of cleaning validations have recently changed.

In the past, validations of cleaning processes were performed based on recommendations found in AAMI TIR30 as well as the FDA guidance document concerning labeling and validation methods for reusable devices. Late in August 2022, AAMI TIR30 was replaced by a new standard, ANSI/AAMI ST98 “Cleaning validation of health care products – Requirements for development and validation of a cleaning process for medical devices”.
ANSI/AAMI ST98 evolved from AAMI TIR30 out of a need to provide requirements for performing cleaning validations due to the increased complexity of medical devices, emergence of new pathogens, and scientific advancement in the processing of medical devices. This new standard affects how cleaning validations of all reusable medical devices will be performed going forward, ranging from non-critical to critical devices. As of now, it is unclear what kind of grace period will be put in place to comply with the new standard. For legacy or recently validated products, manufacturers can perform a gap analysis to identify any additional testing or assessments that may be needed to meet the current standard requirements.
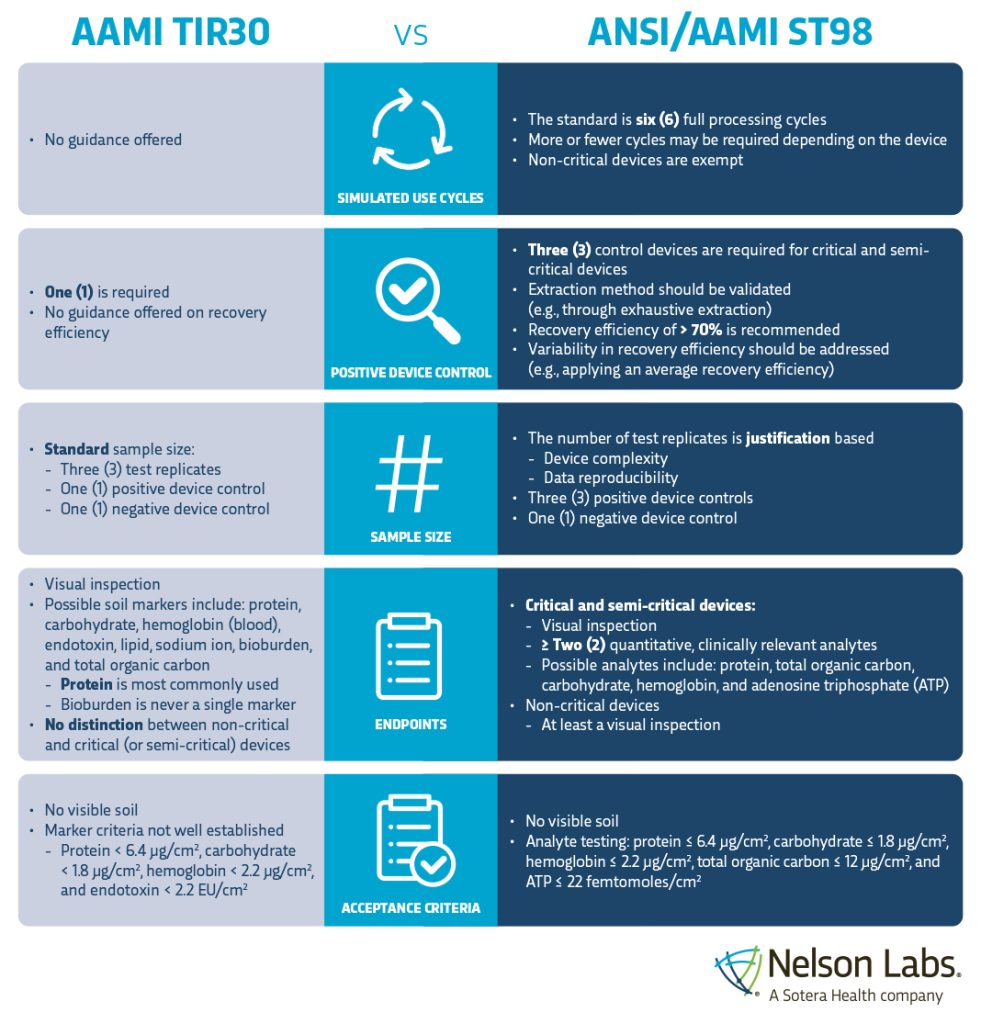
Nelson Labs has experts actively serving on the relevant ISO and AAMI committees that can help every step of the way, from identifying gaps to performing the actual validations in our ISO17025- and GLP-accredited laboratories. To further aid our customers in the transition from AAMI TIR30 to ANSI/AAMI ST98, we have summarized the most significant changes in the infographic below.
As always, Nelson Labs has a dedicated and experienced team of experts to help you understand the latest testing requirements and with whom you can consult about any of your product-family testing needs. We also have world-class laboratories to perform the testing you need. Contact us at [email protected].