What is the Zone of Inhibition Test?
The Zone of Inhibition Test (also known as the Kirby-Bauer Test, Antimicrobial Susceptibility Test, Disk Diffusion Test, or the Agar Diffusion Test) is a quick and inexpensive way to assess the antimicrobial activity of a material or solution in relation to a target microorganism. Zone of Inhibition testing has been utilized for years. It was first developed in the 1950’s and then standardized by the World Health Organization in 1961.
The Zone of Inhibition Test is a basic microbiological test commonly used throughout the medical device and pharmaceutical industries. Its quick analysis allows for screening an antimicrobial product for inhibitory activity against a panel of different microorganisms. It is best performed if the antimicrobial can leach out of the material, with tested materials typically including leachable antimicrobials that has been embedded in plastics, textiles, solids, surfaces, liquids, gels, etc. as well as antibiotic solutions. Guidance for the susceptibility test from a clinical perspective may be found in:
- CLSI Supplement M100 “Performance Standards for Antimicrobial Susceptibility Testing”
Why is Zone of Inhibition Testing Important?
This method of testing is simple and more affordable than expensive alternatives like the broth or agar dilution methods. Additionally, Zone of Inhibition testing is a more rapid way to determine the performance of a specific antimicrobial agent. In this method, several samples can be tested for antimicrobial properties swiftly with results can available between a few days and 2-3 weeks. As such, this testing is frequency used as a preliminary screening of antimicrobial properties and often used in product development phases.
At Nelson Labs, we provide technical expertise accompanied by proficiency in testing a vast catalog of products and bacterial assessments. Our Zone of Inhibition Testing is executed accurately and knowledgeably.
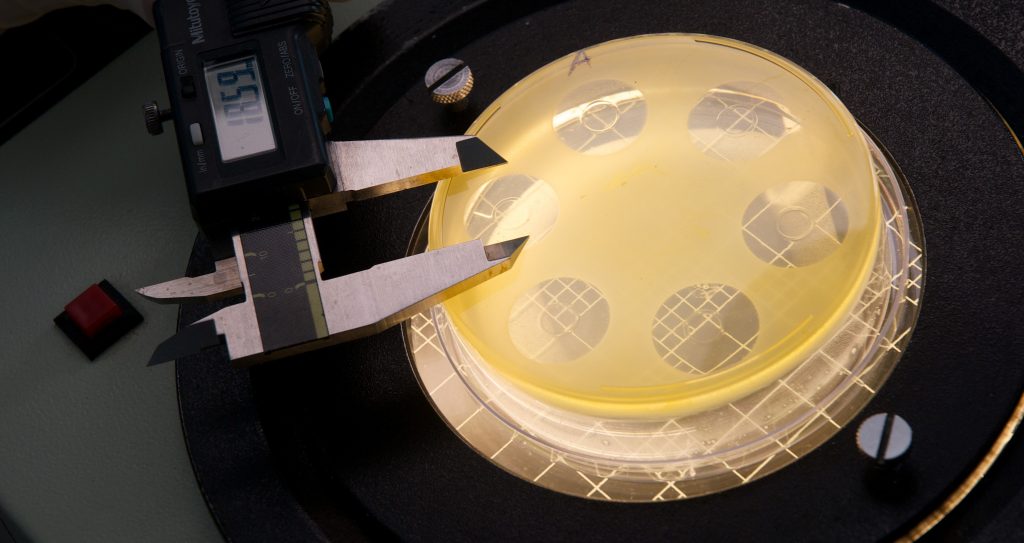
In order to perform the Zone of Inhibition, Disk Diffusion Test, an agar plate is thoroughly spread with bacteria or fungal cells using a sterile swab. The antibiotic of choice is then infused into small paper filter discs. The antibiotic-soaked filter discs are placed strategically on the agar plate and the whole plate is incubated for 18-24 hours. The results are visible immediately after incubation, with large “zones of inhibition” appearing around the discs indicating that the antibiotic is effective against the present bacterial or fungal strain. If no “zone of inhibition” is present, then there is an indication for resistance against the antibiotic. The size of the diffusion between the filter discs and the cells usually refers to the level of antimicrobial activity present in the sample or product. The “zone of inhibition” is measured, documented, and interpreted by our experienced scientists and quickly send to the manufacturer.